Most plastics are produced from petrochemicals. Motivated by the finiteness of oil reserves and threat of global warming, bio-plastics are being developed. These plastics degrade upon exposure to sunlight, water or dampness, bacteria, enzymes, wind erosion and in some cases pest or insect attack, but in most cases this does not lead to full breakdown of the plastic. When selecting materials, designers must consider the moral, ethical and environmental implications of their decisions.
Early plastics used from 1600 BCE through to 1900 CE were rubber based. Prompted by the need for new materials following the first world war, the invention of Bakelite and polyethylene in the first half of the 20th century sparked a massive growth of plastic materials and as we identify the need for new materials with particular properties, the development of new plastics continues.
Development of Plastic
- Early plastics were rubber based
- After the 1st World War a need for new materials which led to the invention of Bakelite and polyethylene.
- WWII saw a need for new materials such as plastics, e.g. nylon to replace silk for parachutes.
- Some uses of plastic during the war are now in everyday use.
- Read this BBC article on the history of plastics
- BBC on plastics
Raw materials for plastics
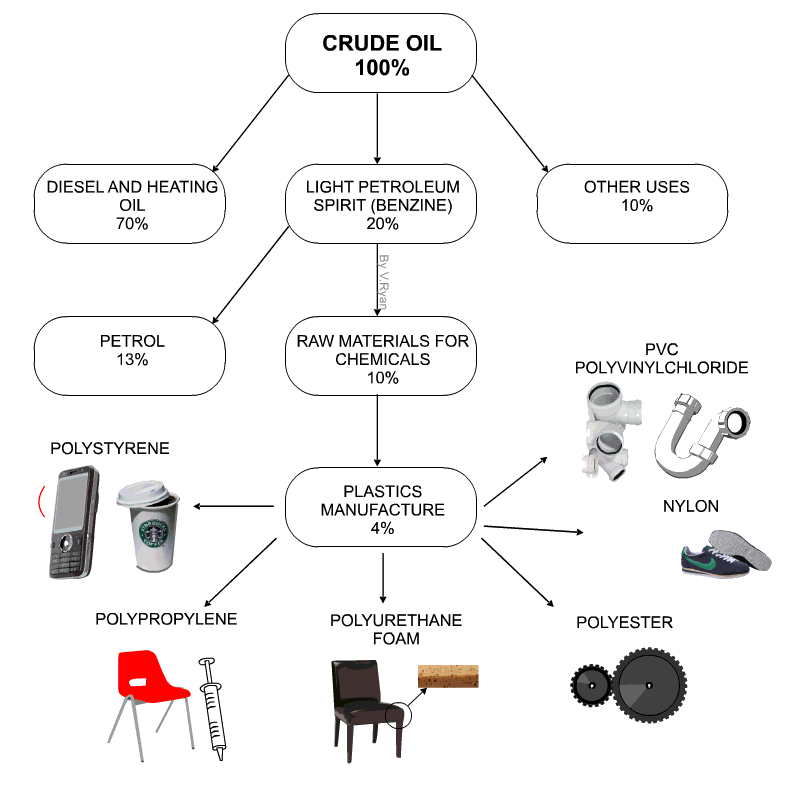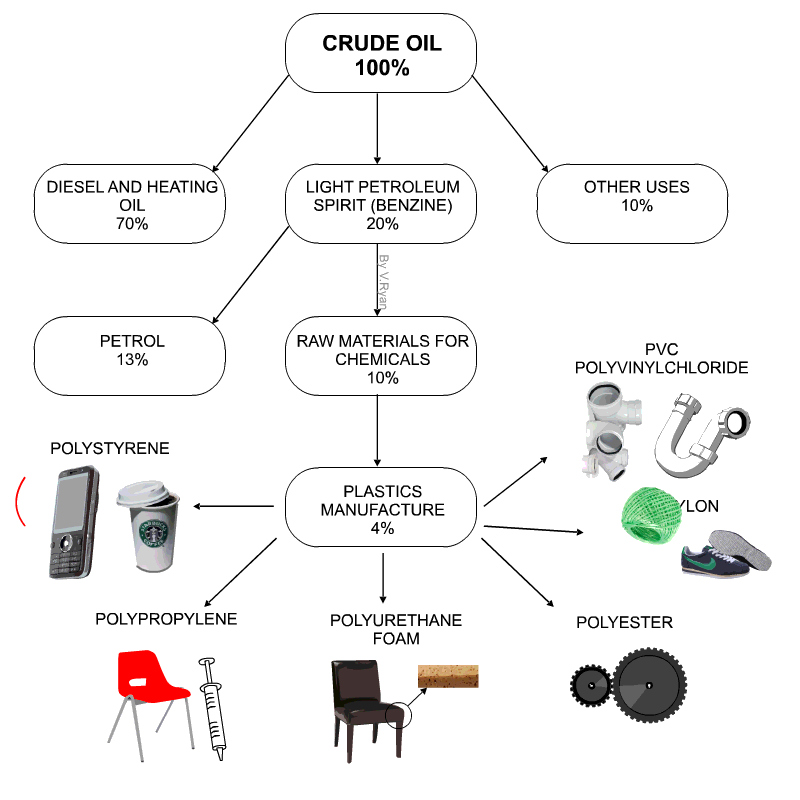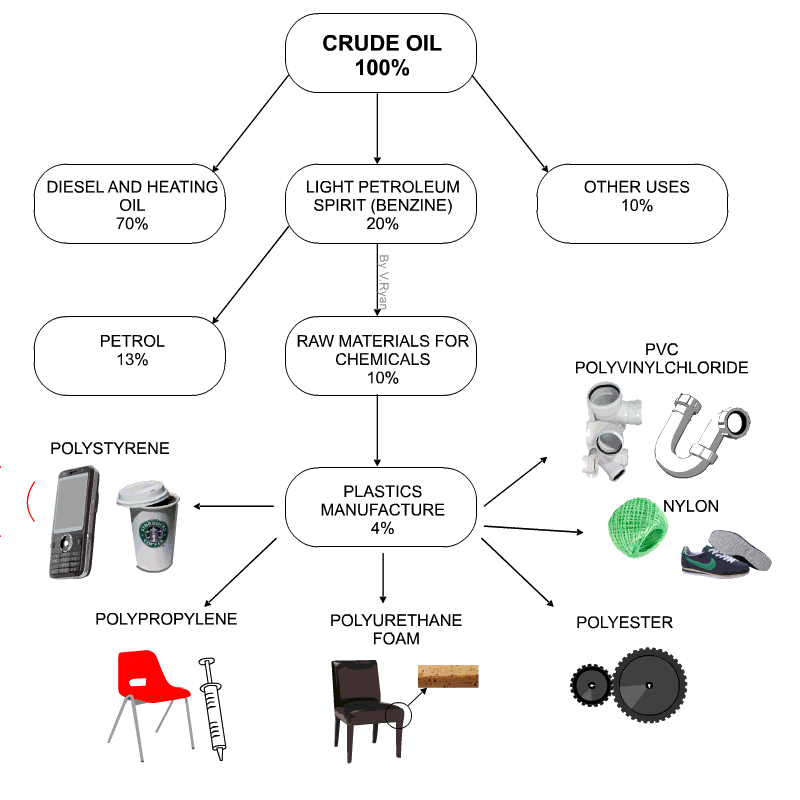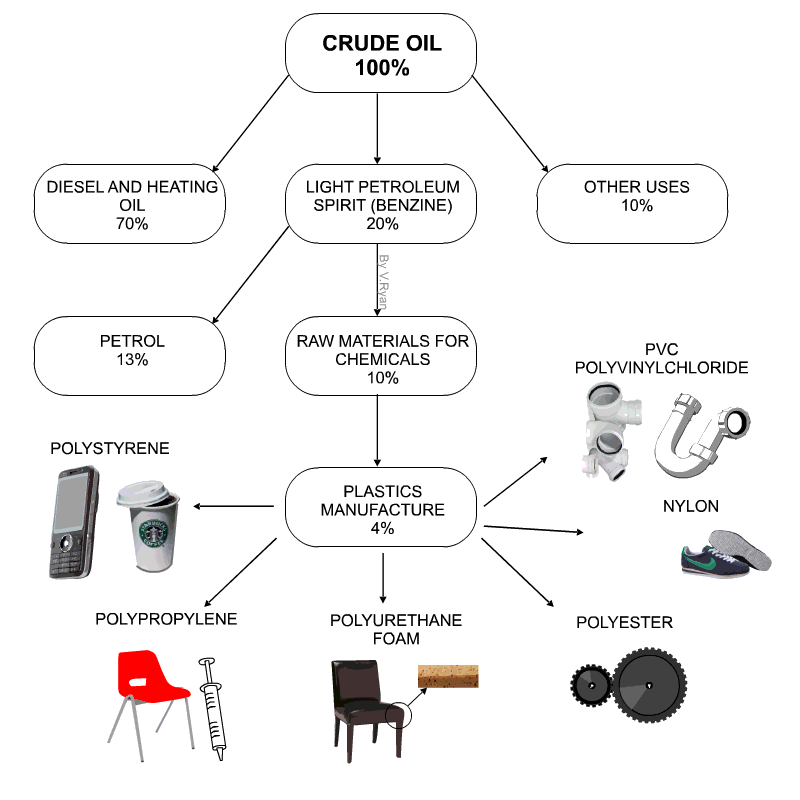
- Technically plastics are referred to as Polymers
- Petro-chemicals are the main ingredient for modern day plastics.
- Roughly 8% of oil production is due in plastics
- The raw material for plastics (mainly oil) is extracted in a country, exported to other countries where conversion to plastics takes place and these are re-exported at considerable added value. (Int Mind)
- Bio-plastics – such as polylactic acid (PLA) resin which is made from corn or potato starch – is also biodegradable.
- Interesting note on compostability of PLA
Structure of thermoplastics
- Thermoplastics are linear chain molecules with weak secondary bonds between the chains.
- The Making of the Modern World has an informative article on Polymers and intermolecular bonds
- Tecnoelpalo on Plastics including their uses.
- General characteristics include:
- ductile
- low stiffness – squishy water bottles for example
- easily injected into a mould
- can be reshaped after heating
- easily and cost effectively manufactured
| Types of thermoplastics | Properties | Design Context | |
|
PP (Polypropene) |
|
Buckets, bowls, crates, toys, food containers, juice bottles, straws, coat hangers, furniture eg plastic chairs), | |
|
PE (Polyethene) |
|
Water bottles, food storage containers, plastic tubing, light work surfaces, shampoo bottles, flower pots | |
|
Soda and water bottles, clam-shelll food containers, fibers (eg polyester), parts made by injection moulding, | ||
|
telephone handsets, rigid luggage, domestic appliances, handles, some helmets (impact), product prototyping, | ||
|
HIPS (High Impact PolyStyrene) |
|
toys, kitchen utensil, shelves, signs, product casings. | |
|
PVC (Polyvinyl chloride) |
|
potable piping, water bottles, cling film, credit cards, toys, shampoo bottles, imitation leather, window frames, rain gutters, garden furniture, binders and even pens – as a wide use |
‘Sam Suds’ Courtesy of Center for Health, Environment and Justice
Structure of thermosetting plastics
- Thermosets are linear chain molecules with strong primary bonds between adjacent polymer chains.
- This gives thermosets a rigid 3D structure.
- Characteristics include:
- high stiffness
- higer strength than thermoplastics
- cannot be reheated and remoulded – it will usually char
Properties of polyurethane, urea-formaldehyde, melamine resin and epoxy resin
| Type of thermoset | Properties | Design Contexts | |
|
wheels, wing parts, sponges, finishes (lacquer etc), toys, | ||
|
manufacture of man-made timber (pressed) products such as MDF particle board, adhesives, insulation, wrinkle and shrink resistant textiles | ||
|
kitchen ware (plates, utensils), decorative laminates (counter or table tops), fire retardant clothing, | ||
| Epoxy resin |
|
adhesives, fibre optics, electrical insulators and transformers, craft and jewelry industry |
Recovery and disposal of plastics
- Nearly all plastics can be recycled it mostly depends on economic, technical and logistical factors
- Thermoplastics can be easily recycled.
- come in a range of chemical compounds and therefore need to be sorted for recycling
- Thermosets not so easy (and expensive to do so)
- need to be ground into a powder which adds time and costs.
- Often get sent to landfill
- Some plastics are not accepted at the recycling plant due to its chemical compound and the facilities capabilities.
Temperature and recycling thermoplastics
- The Increase in temperature causes the weak secondary bonds to break (The heat is sufficient enough to break the secondary bonds but not the primary, covalent, bonds) see above image.
- This allows the long molecular chains to slide over each other, i.e. be reshaped into a new product.
- When a plastically deformed and allowed to cool it will remain in the new shape due to new secondary bonds being formed.
- The nature of the structure of a thermoplastic allows it to be readily recycled.

Something Extra …

